Effectiveness
Confirmed high efficiency in the diagnosis of SARS CoV-2.

Medical diagnostics
Confirmed high accuracy
The assessment of usefulness, sensitivity and specificity confirmed in clinical conditions by the Clinical Research Center of CM Medyk, which has so far conducted over 145 clinical trials (phases Ia, II, III, IV), incl. for Astra Zeneca, Johnson & Johnson, Bayer, Roche, GSK, Teva, PPDI, Scope. The center has undergone many audits, including the audit of the American FDA (Food and Drug Administration), obtaining the highest ratings.
700 patients were examined, of which 155 measurements were used to parameterize the device.
The effectiveness of the tests is in line with the recommendations of the WHO, which recommends the use of rapid antigen tests, which are characterized by specificity ≥ 97% and sensitivity ≥ 80% compared to the genetic method.
When analyzing the obtained results, the relationship between the viral load and the obtained results is clearly visible. The more pathogen in the collected material, the higher the sensitivity of the device.
The results of the research are consistent with the position of the board of the Polish Society of Epidemiologists and Doctors of Infectious Diseases on the diagnostic value of antigen tests used in the diagnosis of SARS-CoV-2 infections as of November 14, 2020 and the World Health Organization (WHO), which recommends the use of rapid antigen tests, which are characterized by a sensitivity of ≥ 80% and a specificity of ≥ 97% compared to the genetic method.
Citing the recommendations regarding antigen tests is a result of the lack of such recommendations for devices for mass diagnosis of SARS-CoV-2 virus detection, among others in exhaled air which is the innovative Covid Detector device. Especially considering the advantages of the device such as measurement speed, simple operation, efficiency and sensitivity comparable to good antigen tests.
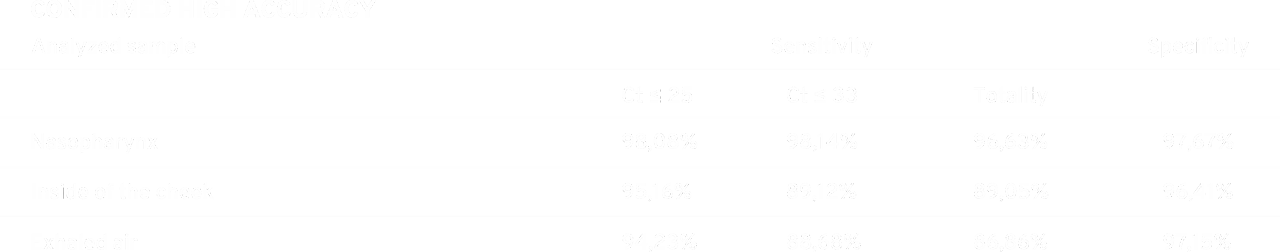
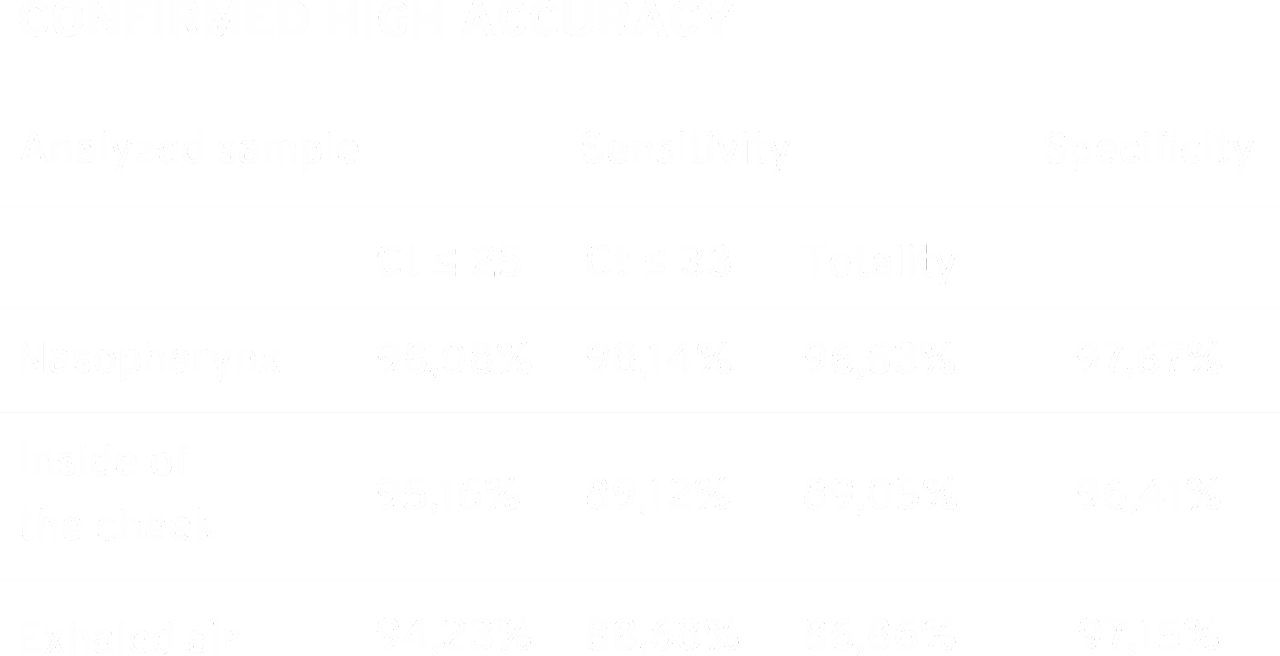
The results obtained during the control tests of the swabs confirm the effectiveness of the device in detecting the SARS-CoV-2 virus. The achieved results of specificity of 97.67% for nasopharynx and 96.41% for cheek swabs, and sensitivity of 96.63% for nasopharyngeal swabs and 89.05% for buccal swabs indicate the high diagnostic possibilities of the device regardless of the type of diagnostic material.
When analyzing the obtained results, the relationship between the viral load and the obtained results is clearly visible. The more pathogen in the collected material, the higher the sensitivity of the device.
The results of the research are consistent with the position of the board of the Polish Society of Epidemiologists and Doctors of Infectious Diseases on the diagnostic value of antigen tests used in the diagnosis of SARS-CoV-2 infections as of November 14, 2020 and the World Health Organization (WHO), which recommends the use of rapid antigen tests, which are characterized by a sensitivity of
≥ 80% and a specificity of ≥ 97% compared to the genetic method.
Citing the recommendations regarding antigen tests is a result of the lack of such recommendations for devices for mass diagnosis of SARS-CoV-2 virus detection, among others in exhaled air which is the innovative Covid Detector device. Especially considering the advantages of the device such as measurement speed, simple operation, efficiency and sensitivity comparable to good antigen tests.
The results obtained during the control tests of the swabs confirm the effectiveness of the device in detecting the SARS-CoV-2 virus. The achieved results of specificity of 97.67% for nasopharynx and 96.41% for cheek swabs, and sensitivity of 96.63% for nasopharyngeal swabs and 89.05% for buccal swabs indicate the high diagnostic possibilities of the device regardless of the type of diagnostic material.
